Preparation and Stability of Waterborne Polyurethane Hydroxyl Component
Liu Xuemei, Zhang Liangjun, Fan Qingchun
(Key Laboratory of Ministry of Education, Ministry of Education, Ministry of Education, Wuhan University of Technology, Wuhan 430073, China)
Abstract: Aqueous polyurethane hydroxy group with branched structure was synthesized by using polypropylene glycol (N210), 2,4-toluene diisocyanate (TDI), dimethylolpropionic acid (DMPA) and trimethylolpropane (TMP) as main raw materials. Component. The effect of process conditions on the content of NCO in hydroxyl component was investigated. The product was characterized by infrared spectroscopy (IR). The particle size of the emulsion was tested by submicroscopic visualization reaction device. The emulsion was determined by Stokes free sedimentation rate equation. Settling rate. The results showed that the suitable reaction conditions were reaction temperature of 75 °C and reaction time of 4 h. The stability of the emulsion was closely related to the content of DMPA and TMP. The emulsion stability increased with the increase of DMPA content. When the mass fraction of TMP was 0~ At 4.7%, the sedimentation rate is (0.041×10-13)~(2.124×10-13) m/s, and the emulsion is stable.
Key words: waterborne polyurethane; visualization; Stokes equation
1 Introduction
With the rapid development of the world's industry, environmental pollution has become more and more serious, and it has reached the point of affecting the sustainable development of the economy. A large amount of organic volatiles (VOC) are used in conventional coating products, and VOC is volatilized into the atmosphere after construction, causing atmospheric pollution. Water-based paints use water as the main dispersion medium instead of organic solvents, and are rich in water resources, cheap and easy to obtain, non-combustible, and superior in superiority. Therefore, the investment in research and development of water-based coatings is larger than that of other environmentally friendly coatings, and it has also promoted its development [1, 2]. Waterborne polyurethane coatings account for a large proportion of waterborne coatings and are an important coating product. Because it not only has the non-polluting effect of water-based paints, but also has excellent weather resistance and similar performance to solvent-based coatings, it is an excellent environmentally friendly coating. Therefore, it has attracted the attention of researchers at home and abroad [3-6]. In this paper, an aqueous polyurethane hydroxy component with a crosslinked structure was synthesized, which can be used to formulate an aqueous polyurethane two-component coating with excellent performance.
2. Test section
2.1 Raw materials and their treatment
Polypropylene glycol (N210): industrial product, Fushun Jiahua Polyurethane Co., Ltd., under N2 atmosphere, vacuum dehydration at 100 °C for 4h, 4A molecular sieve for use; 2,4-toluene diisocyanate (TDI): analytically pure, Shanghai Reagent First Factory; Dimethylolpropionic acid (DMPA): supplied by Guangdong Huarun Coating Co., Ltd., vacuum dehydrated at 100 °C for 4 h, placed in a desiccator for use; triethylamine: analytically pure, Shanghai Reagent No. 1; Trimethylolpropane (TMP) : Chemical Pure (CP), Shanghai Pharmaceutical Reagent Company of China Pharmaceutical Group, formulated with 50% acetone solution, 4A molecular sieve for storage; Acetone: Analytically pure, Tianjin Dongli District Tianda Chemical Reagent Factory; Polyisocyanate curing agent, solid The content is 100%, Wuhan Shiquanxing Decorative Coatings Co., Ltd.
2.2 Synthesis of waterborne polyurethane hydroxyl component
Under the protection of dry nitrogen, an appropriate amount of N210, DMPA, TMP and acetone were added to a 250 mL four-necked flask equipped with a thermometer, a stirrer and a reflux condenser. After heating and dissolved completely, the temperature was stabilized at 65 ° C, and TDI was started to be added dropwise. , 1h drop. After heating to 75 ° C for 4 h, an aqueous polyurethane prepolymer was obtained. After cooling to 52 ° C, triethylamine was added dropwise, and then deionized with water, and acetone was removed under reduced pressure to obtain a translucent aqueous polyurethane component having a pale blue fluorescence. The synthetic reaction steps are shown in Figure 1.
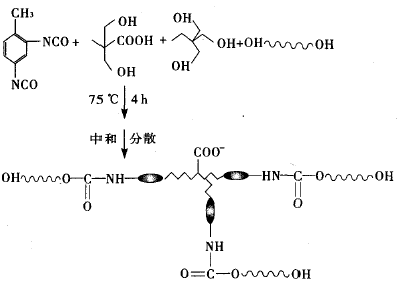
Figure 1. Synthesis process of aqueous polyurethane hydroxyl component
2.3 Structural characterization and performance testing
(1) Infrared spectroscopy
Infrared spectroscopy (IR) was tested with a Nicolet FT-IR670 infrared spectrometer.
(2) Determination of NCO content [7]
Accurately weigh 1.0 g of the hydroxyl component into a conical flask, pipette 10 mL of di-n-butylamine solution (V di-n-butylamine: V-butyl acetate = 1:5), and gently shake to make Dissolve and mix well, let stand for 20~30min, add 25mL absolute ethanol and shake well, add a few drops of 0.1% bromocresol green indicator, titrate with 0.5mol/L HCl standard solution until it just becomes Yellow is the end point and a blank test is done at the same time.
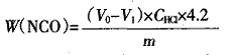
Where: V0 is the volume of the HCl standard solution consumed by the blank test, mL; V1 is the volume of the sample HCl standard solution, mL; CHCl is the concentration of the HCl standard solution, mol/L; m is the mass of the sample, g.
(3) Visual instrument
The test instrument is a miniature visualization reactor that can observe the motion state of micron-sized particles [8].
(4) Determination of density
The aqueous polyurethane hydroxy component is an emulsion obtained by measuring the density of the liquid [9].
3. Results and discussion
3.1 Infrared spectroscopy
The infrared spectrum of the two components of the two-component waterborne polyurethane lacquer and its coating film is shown in Fig. 2.
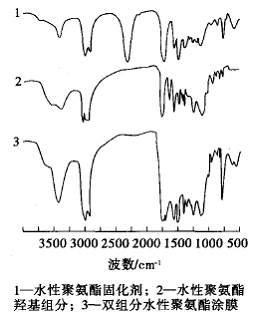
Figure 2 Infrared spectra of two components and coating film of two-component waterborne polyurethane paint
It can be seen from curve 1 of Fig. 2 that 2272.12 cm-1 is a characteristic absorption peak of the -NCO group. It can be seen from curve 2 that the overlap absorption peak of -OH and -NH at 3305.66cm-1, and the characteristic absorption peak of CO in the carbamate group at 1728.72cm-1, 1534.69cm-1 and 1228.16cm-1 are N- H deformation, C-N stretching vibration absorption peak; 1601.65cm-1, 1536.69cm-1, 780.67cm-1 is the characteristic absorption peak of the benzene ring. It can be seen from curve 3 that the absorption peak of NCO disappeared at 2672.12 cm-1, indicating that a crosslinking reaction occurred between the NCO and -OH groups.
3.2 Influence of process conditions
3.2.1 Effect of reaction temperature
In the synthesis of the hydroxyl component, the reaction temperature has a great influence on its structure and properties. It was found that when the temperature reached 85 ° C, the viscosity of the hydroxyl component became too large and gelatinized within 2 h; however, the reaction temperature was too low, and the preparation of the hydroxyl component was too long and the energy consumption was large. Therefore, it is important to choose the right reaction temperature.
3.2.2 Effect of reaction time
The reaction temperature is high, the time required to reach the same -NCO content is short, and the reaction temperature is low, which takes a long time. Figure 3 is a graph showing the relationship between the reaction time and the NCO content in the hydroxyl component at different temperatures.
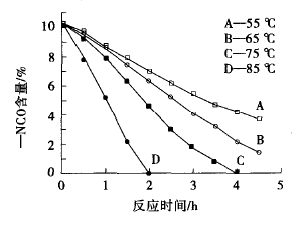
Figure 3 Effect of reaction time on the content of a NcO in the hydroxyl component
It can be seen from Fig. 3 that the reaction at 55 ° C and 65 ° C for 4.5 h, the - NCO content of the system is 3.7% and 1.5%, respectively, it takes a long time to complete the reaction, the energy consumption is large; the reaction at 75 ° C for 4 h, - NCO The content was 0, and the reaction was complete; however, when the temperature rose to 85 ° C, the condensation occurred, and the reaction was complete at 2 h, but the viscosity of the system was very large, and a gel appeared. Therefore, it is suitable to select a reaction temperature of 75 ° C and a reaction time of 4 h.
Effect of 3.3 DMPA content on emulsion stability
3.3.1 Visualization of microemulsion and determination of particle size
Five groups of emulsions (DMPA content of 3.1%, 4.3%, 5.0%, 6.7%, 8.3%, respectively) were diluted 1:100 with double distilled water, and treated with ultrasonic for 10 minutes, respectively, and then loaded into a quartz cuvette. After the microscopic visualization reactor observation system is turned on, the sample is placed in the observation area and a suitable angle of view is found, and the motion state of the emulsion particles is observed. The photograph is shown in Fig. 4.
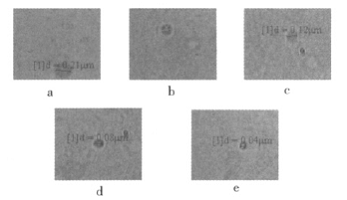
Figure 4 Microscopic visible photos of 5 groups of emulsions with different DMPA content
It can be seen from Fig. 4 that the DMPA content increases from 3.1% to 8.3%, and the particle diameter dp decreases from 0.21 μm to 0.04 μm, indicating that the particle size of the emulsion becomes smaller as the DMPA content increases.
3.3.2 Describe the emulsion sedimentation process using the Stokes free sedimentation rate equation
Stokes free settlement rate equation [10]:
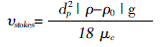
Where: dp is the particle size of the emulsion, Ï and Ï0 are the density of water and latex particles, respectively, and μc is the viscosity of water. The density of water is known to be Ï = 1 g/cm3, and the viscosity of water at 25 ° C is μc = 1.5188 mPa·s. The dp and Ï0 are listed in Table 1, and the emulsion settling rate is determined according to the Stokes equation.
It can be seen from Table 1 that as the DMPA content increases, the sedimentation rate of the emulsion becomes smaller and smaller, and the emulsion becomes more and more stable. This indicates that the stability of the emulsion is related to the content of DMPA. Structurally, the ionic group in the polyurethane structure is a hydrophilic group formed by forming a salt of a carboxyl group on the side chain of the polymer with triethylamine, which allows the polyurethane to be uniformly dispersed in water. The greater the content of DMPA, the stronger the hydrophilicity of the molecule and the better the dispersibility. Too low a content of DMPA does not form a stable emulsion. Therefore, the more the DMPA is used, the more stable the emulsion is.
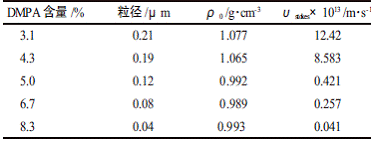
Table 1 Effect of DMPA content on emulsion settling rate
3.4 Effect of TMP content on emulsion stability
3.4.1 Visualization of microemulsion and determination of particle size
Five groups of emulsions (TMP content of 0, 1.1%, 3.1%, 4.7%, 6.3%) were diluted 1:100 with double distilled water, and treated with ultrasonic for 10 min, respectively, and then loaded into a quartz cuvette. After the microscopic visualization reactor observation system is turned on, the sample is placed in the observation area and a suitable viewing angle range is found, and the movement state of the emulsion particles is observed. The photograph is shown in Fig. 5. It can be seen from Fig. 5 that the TMP content increases from 0 to 6.3%, and the particle diameter dp increases from 0.04 μm to 0.36 μm, indicating that the particle size of the emulsion increases with the increase of the TMP content.
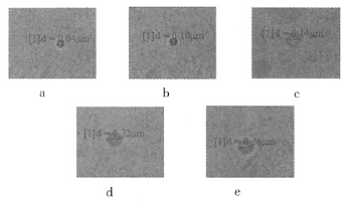
Figure 5 Microscopic visible photos of 5 groups of emulsions with different TMP contents
3.4.2 Describe the emulsion settling process using Stokes free sedimentation rate equation
The emulsion settling rate was determined according to the Stokes equation, see Table 2.
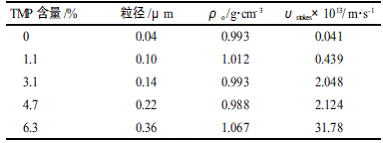
Table 2 Effect of TMP content on dispersion properties
It can be seen from Table 2 that as the TMP content increases, the sedimentation rate of the emulsion becomes larger and larger, and the emulsion stability becomes worse and worse. Because TMP is a trifunctional polyol, its content increases, the average functionality of the system increases, and the particle size of the polycondensation product increases [11], so the TMP dosage is preferably 0 to 4.7%.
4. Conclusion
The aqueous polyurethane hydroxy component with branched structure was synthesized. The suitable process parameters were obtained: the reaction temperature was 75 ° C and the reaction time was 4 h. The more DMPA content, the more stable the emulsion, the mass fraction of TMP was 0-4.7%. The emulsion is relatively stable.
Small Padlocks,Double Keys Padlock,Steel Keyed Padlock
Lockout Station And Lockout Kit Padlock Co.,Ltd , http://www.chpadlock.com