Major progress in the development of cathode catalysts for proton exchange membrane fuel cells
Recently, Prof. Zeng Jie from the Hefei National Laboratory for Physical Sciences at the Microscale and the School of Chemistry and Materials Science at the University of Science and Technology of China, cooperated with Prof. Peng Zhenmeng from Akron University and Prof. Rui from Shanghai Institute of Applied Physics, and developed a cathode catalyst for proton exchange membrane fuel cells. Significant progress has been made. Based on the ensemble effect, the researchers designed a ruthenium-doped platinum ultrafine nanowire catalyst that exhibits high activity and high stability in the cathode oxygen reduction of fuel cells. This work is entitled "Achieving Remarkable Activity and Durability toward Oxygen Reduction Reaction Based on Ultrathin Rh-Doped Pt Nanowires" and published in the Journal of the American Chemical Society (J. Am. Chem. Soc. 2017, 139, 8152- 8159), the co-first author of the paper is postdoctoral Huang Hongwen and doctoral candidate Li Wei.
Oxygen Reduction Reaction of Ultrafine Nanowires with Coated Phenanthrene
Proton exchange membrane fuel cells (PEMFCs) are considered to be promising clean energy conversion technologies for efficient power delivery of transportation vehicles and mobile devices. However, the cathodic oxygen reduction reaction requires a large amount of precious metal platinum as a catalyst due to the slow kinetics, which increases the manufacturing costs of related parts, thereby restricting the commercialization of the technology. Increasing the mass activity of the platinum catalyst in the oxygen reduction reaction can effectively reduce the amount of platinum, thereby achieving cost reduction. Catalysts for increasing platinum utilization in catalysts have emerged in an endless stream. Many reported platinum-based catalysts have excellent mass activity, but the stability of most of them is not significant. The lack of stability of these platinum-based catalysts is mainly due to the fact that the structures on which the high-quality activity is dependent cannot stably exist in thermodynamics, and therefore cannot have both high-quality activity and excellent stability.
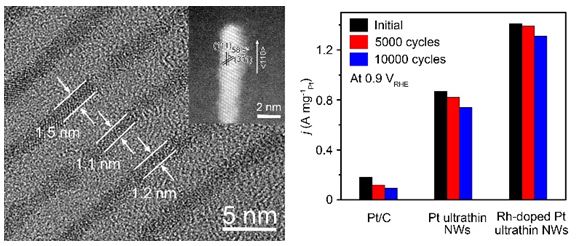
Faced with this challenge, researchers have introduced helium atoms to enhance their stability while adjusting the dimensions of the platinum-based catalyst to change the symmetry and the contact area with the carbon load. The germanium atom-doped platinum ultrafine nanowires have a diameter of only 1.3 nanometers, and the platinum atom utilization rate is as high as 48.6%. The mass activity and specific activity of carbon-supported germanium atom-doped platinum-based ultrafine nanowires were 7.8 and 5.4 times higher than those of commercially available platinum carbon catalysts. At the same time, the catalyst was only 9.2% after 10,000 cycles in an oxygen atmosphere. Loss of mass-activity performance, while the commercial platinum carbon catalyst used in the process was recirculated for 10,000 times in an oxygen atmosphere, the loss in mass activity performance reached 72.3%.
The study was supported by projects of the Chinese Academy of Sciences forefront scientific research projects, national major scientific research projects, the National Natural Science Foundation, and postdoctoral science foundation projects.
Horizontal Multistage Centrifugal Pump
Horizontal Multistage Centrifugal Pump,Fire Pump,Horizontal Multi-Stage Pump,Water Boosters Pumps
Shanghai NuoSai Pump Manufacturing Co., Ltd. , https://www.nuosai.net